Press Releases

Qihan Biotech Initiates Dosing in Phase I/IIa Clinical Trial with Off-the-Shelf Allogeneic CAR-T Therapy for Refractory Systemic Lupus Erythematosus
December 2025
QihanBiotech

Qihan Biotech Presents New Clinical Data on Allogeneic CAR-T Cell Therapies and Preclinical Data on In Vivo CAR-T at the American Society of Hematology (ASH) Annual Meeting
December 2025
Qihan Biotech presented an oral and several poster presentations at the 67th American Society of Hematology (ASH) Annual Meeting.

Qihan Biotech Announces Multiple Presentations at the 2025 American Society of Hematology (ASH) Annual Meeting
November 2025
At the upcoming 2025 ASH Annual Meeting, Qihan Biotech will present new data highlighting significant progress across its off-the-shelf and in vivo CAR-T cell therapy programs.

Qihan Biotech Announces FDA Clearance of IND for QT-019B, a Universal Dual-Target CAR-T Cell Therapy for Refractory Systemic Lupus Erythematosus
August 2025
The U.S. Food and Drug Administration (FDA) has cleared its Investigational New Drug (IND) application for QT-019B.
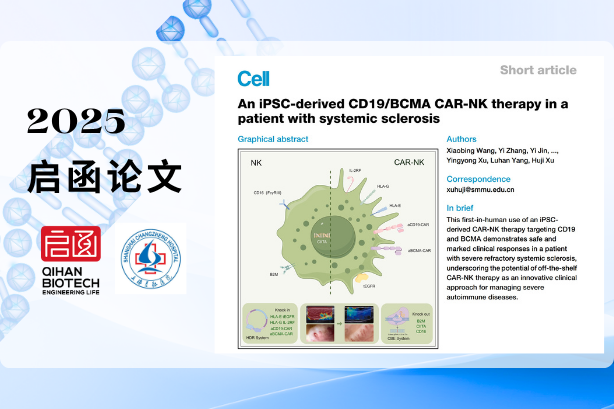
iPSC-CAR-NK Cell Therapy Clinical Results Published in Cell, Marking a Global Breakthrough in Autoimmune Disease Treatment
June 2025
Qihan Biotech announced the publication of groundbreaking clinical results in Cell.

Qihan Biotech Presented its Breakthrough CAR-T Research at ASGCT 2025
May 2025
QihanBiotech announced new advancements in its universal CAR-T cell therapy research, to be presented at the 28th Annual Meeting of the American Society of Gene & Cell Therapy (ASGCT).

Qihan Biotech Announces Appointment of Carl June to Scientific Advisory Board
December 2024
Qihan Biotech is proud to announce the appointment of Dr. Carl June as Cell Therapy Chair of its Scientific Advisory Board.

Qihan Biotech Announces Publication in Microbiology Spectrum of CRISPR-Cas Multiplexed Genome Editing for Reducing African Swine Fever Virus
May 2024
Qihan Biotech announced its groundbreaking research titled "Testing Multiplexed anti-ASFV CRISPR-Cas9 in Reducing African Swine Fever Virus" in Microbiology Spectrum, an academic journal of the American Society for Microbiology.

Qihan Biotech Appoints Yingyong Xu to its Chief Medical Officer
May 2024
Qihan Biotech today announced the appointment of Mr. Yingyong Xu as the Chief Medical Officer.
- 1
- 2
- 3